
a)
Interpretation: Boiling point trends that are highlighted below should be determined.
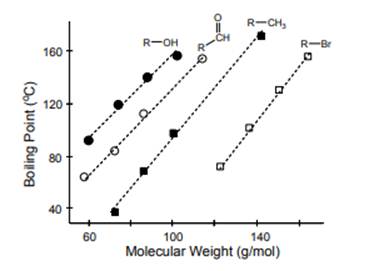
Concept introduction: Boiling is used to define when a substance gets converted from its liquid to the vapor phase at the boiling point. The temperature where the vapor pressure of the liquid becomes equal to surrounding or atmospheric pressure is called the boiling point. It depends on the strength of intermolecular forces. Stronger the intermolecular forces, higher will be the boiling point of the molecule and vice-versa. The magnitude of the boiling point increases with an increase in molecular mass and surface area.
b)
Interpretation: Reason for
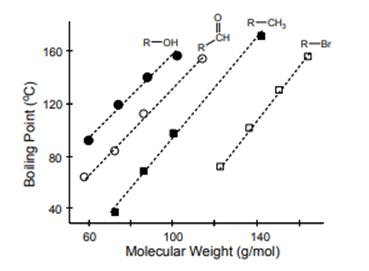
Concept introduction: Boiling is used to define when a substance gets converted from its liquid to the vapor phase at the boiling point. The temperature where the vapor pressure of the liquid becomes equal to surrounding or atmospheric pressure is called the boiling point. It depends on the strength of intermolecular forces. Stronger the intermolecular forces, higher will be the boiling point of the molecule and vice-versa. The magnitude of the boiling point increases with an increase in molecular mass and surface area.
c)
Interpretation: Molecular property that is plotted along
Concept introduction: Boiling is used to define when a substance gets converted from its liquid to the vapor phase at the boiling point. The temperature where the vapor pressure of the liquid becomes equal to surrounding or atmospheric pressure is called the boiling point. It depends on the strength of intermolecular forces. Stronger the intermolecular forces, higher will be the boiling point of the molecule and vice-versa. The magnitude of the boiling point increases with an increase in molecular mass and surface area.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Organic Chemistry: A Guided Inquiry
- Consider an oxygen atom contained in a neutral molecule, like those in Model 1. Give the name for the electrons around the oxygen that are not involved in a covalent bond. What bond angle is formed when oxygen forms two single bonds? What shape is formed? Which functional groups in Model 1 are polar? When a carbon atom forms a double bond to an oxygen atom, it is called a carbonyl group. Which functional groups in Model 1 contain a carbonyl group?arrow_forwardThis one right here number 11arrow_forward1. _________ FeBr3 + ______H2SO4 -----> _____Fe2(SO4)3 + ____HBr Show work on how you solve this.arrow_forward
- (C2) Hello! I just want to ask for help whether the answers in the given pictures are correct. If it's not, please help me recheck and resolve it. Please refer to the given pictures below for the answers and questions. Please read the instructions and directions very carefully. Double and triple check your answers, previous tutors got it wrong. NOTE: Type only your answers. Please do not handwritten your answers. Make sure your formulas, solutions and answers' format are all correct.arrow_forwardHow to convert how to convert 1-Hexene to Hexanol using math. Please show work step by step on paperarrow_forwardA Moving to another question will save this response. Question 20 Pataday is the brand name for a type of allergy eye drops. Which of the following is NOT true regarding Pataday? The structure is shown below. HO O contains an ether O contains an amine contains an amide O contains two aromatic rings O contains a carboxylic acid A Moving to another question will save this response. MacB 20 000 000 F2 F3 F4 F5arrow_forward
- Br 1. Mgº, Ether 2. CO₂ 3. H3O+ 1. HBr, H₂O2, hv 2. Mgº, Ether 3. H 4. H3O+ (mild) 5. PCCarrow_forwardII. TRUE or FALSE. Write TRUE if the statement is correct and FALSE if it is wrong. _1. Use of ethers as anesthetics is a vital part of current medical practice. _2. Animals have the ability to produce methanethiol in their intestinal tract due to the action of bacteria on sulfur-containing proteins. _3. The scent of skunks is due to primarily to thioethers. _4. Compound contributing to the "fried onion smell" may include methyl propyl disulfide. _5. The biochemical effects of THC involve sedation, tranquilization, and mild hallucination.arrow_forwardbest answer. Br2 (xs), NaOH (aq) (xs) a) b.) OH Br Br3C CBr3 Br. OHarrow_forward
- A mixture of NH3 and H2 can be separated by applying pressure, because of their high difference in _____. Explain and elaborate.arrow_forwardPlease help me how to solve part a-c. Please show step by step for a better understanding. Thank you very much.arrow_forward4. a - c are correct. Please help with part d.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
