
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 4, Problem 22CTQ
Interpretation Introduction
Interpretation: Hydrogen that can be donated in each strong acid in below table should be encircled.
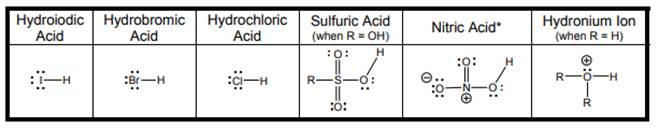
Concept introduction: According to Bronsted-Lowry concept, substance that donates proton is termed as acid while that accepts or gains protons is called base. Species formed after loss of protons from acids are known as their respective conjugate bases whereas conjugate acid is produced by addition of protons to base. Strong acid is substance that donates protons or
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Answer last 3 please (d-f)
can you please help me here? I'll rate if you give the correct answer.
please help - for the last drawing.. (second arrow down) - after this.. please help this answer
Chapter 4 Solutions
Organic Chemistry: A Guided Inquiry
Ch. 4 - Prob. 1CTQCh. 4 - Figure 4.1 is a cartoon depiction of liquid water...Ch. 4 - Prob. 3CTQCh. 4 - Prob. 4CTQCh. 4 - In HF , neither H nor F holds a full formal charge...Ch. 4 - Prob. 6CTQCh. 4 - Prob. 7CTQCh. 4 - Prob. 8CTQCh. 4 - Within any one section of Table 4.2, boiling...Ch. 4 - Prob. 10CTQ
Ch. 4 - Prob. 11CTQCh. 4 - Prob. 12CTQCh. 4 - Prob. 13CTQCh. 4 - Prob. 14CTQCh. 4 - Prob. 15CTQCh. 4 - Prob. 16CTQCh. 4 - Prob. 17CTQCh. 4 - Prob. 18CTQCh. 4 - Prob. 19CTQCh. 4 - Prob. 20CTQCh. 4 - Prob. 21CTQCh. 4 - Prob. 22CTQCh. 4 - (E) Label each of the following as strong acid,...Ch. 4 - Prob. 24CTQCh. 4 - Draw the structure of the conjugate base of water....Ch. 4 - Does Cl have a conjugate acid? If so, what is it?...Ch. 4 - Draw the conjugate base of CH4 (methane).Ch. 4 - For the previous four questions, label each...Ch. 4 - Prob. 29CTQCh. 4 - According to the conventions above, what is the...Ch. 4 - Draw an arrow on Figure 4.13 representing Hrxn4 ....Ch. 4 - Prob. 32CTQCh. 4 - Add a + or above each curved arrow in Figure 4.11...Ch. 4 - Prob. 34CTQCh. 4 - Prob. 35CTQCh. 4 - Prob. 36CTQCh. 4 - Prob. 37CTQCh. 4 - Prob. 38CTQCh. 4 - Prob. 39CTQCh. 4 - Prob. 40CTQCh. 4 - Prob. 41CTQCh. 4 - Prob. 42CTQCh. 4 - Prob. 43CTQCh. 4 - Prob. 44CTQCh. 4 - Prob. 45CTQCh. 4 - Prob. 46CTQCh. 4 - For NH3 (ammonia) and H2O (water)... a. Use curved...Ch. 4 - Prob. 48CTQCh. 4 - Prob. 49CTQCh. 4 - Prob. 50CTQCh. 4 - Prob. 51CTQCh. 4 - Prob. 52CTQCh. 4 - Prob. 53CTQCh. 4 - Prob. 1ECh. 4 - Prob. 2ECh. 4 - Prob. 3ECh. 4 - Prob. 4ECh. 4 - Prob. 5ECh. 4 - Prob. 6ECh. 4 - Prob. 7ECh. 4 - Prob. 8ECh. 4 - Propanal (bp 48°C) and propanol (bp 97°C), both...Ch. 4 - Rank the following molecules from lowest to...Ch. 4 - Prob. 12ECh. 4 - For each molecule below, draw the conjugate acid...Ch. 4 - For each structure you drew in the answer to the...Ch. 4 - Mark each of the following statements True or...Ch. 4 - Organic chemistry is a bit like cooking. Later in...Ch. 4 - Prob. 17ECh. 4 - Prob. 18ECh. 4 - Are endothermic reactions favorable or...Ch. 4 - Prob. 20ECh. 4 - Is bond formation endothermic or exothermic? Write...Ch. 4 - Summarize the relationship between pKa and acid...Ch. 4 - Summarize the relationship between pKa and base...Ch. 4 - Prob. 25ECh. 4 - Consider the following bases: a. For each base...Ch. 4 - Prob. 27ECh. 4 - The following are equivalent ways of asking about...Ch. 4 - Prob. 29E
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following bases: a. For each base above, circle the atom/atoms with the highest PE (will release the most P.E.when a lone pair on this atom combines with an H+ ) b. Rank the bases 1 (highest P.E./strongest base) to 7 (lowest PE/weakest base), and explainyour reasoning.arrow_forward! ( do a with explanation)arrow_forwardHi , can you help me to answer this question .. can you draw curved arrows to move a proton from acid to the base .. Identify acid , base , conjugate acid and conjugate base also draw the product of proton transfer.. I need the full scheme answe so that i can understand this topic better.. Thank youuarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning