
a) Aqueous acidic KMnO4
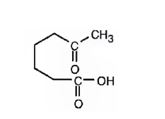
Interpretation:
The product expected when 1-methylcyclohexene reacts with aqueous acidic KMnO4 is to be stated.
Concept introduction:
Potassium permanganate in neutral or aqueous acidic solutions cleaves the double bonds in
To state:
The product expected when 1-methylcyclohexene reacts with aqueous acidic KMnO4.
b)
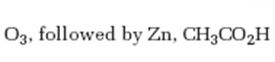
Interpretation:
The product expected when 1-methylcyclohexene reacts with O3, followed by Zn, CH3COOH is to be stated.
Concept introduction:
Ozone adds to the double bond in alkenes to produce compounds called ozonides. The ozonides upon immediate treatment with Zn and acetic acid yield carbonyl compounds. Each carbon in the double bond cleaved gets attached to an oxygen atom. A di-substituted carbon in double bond gives a ketone while a mono-substituted carbon in double bond gives a ketone and an
To state:
The product expected when 1-methylcyclohexene reacts with O3, followed by Zn, CH3COOH.
Trending nowThis is a popular solution!

Chapter 8 Solutions
Organic Chemistry
- What reagents are needed to convert 1-ethylcyclohexene into (a) 1-bromo-2ethylcyclohexane; (b) 1-bromo-1-ethylcyclohexane; (c) 1,2-dibromo-1-ethylcyclohexane?arrow_forwardPredict the major products of the following reactions, (a) pent-1-ene + HCl b) 2-methylpropene + HCl(c) 1-methylcyclohexene + HI (d) 4-methylcyclohexene + HBrarrow_forwardAldehydes and ketones react with thiols to yield thioacetals just as they react with alcohols to yield acetals. Predict the product of the following reaction, and propose a mechanism:arrow_forward
- Nonconjugated , -unsaturated ketones, such as 3-cyclohexenone, are in an acid-catalyzed equilibrium with their conjugated , -unsaturated isomers. Propose a mechanism for this isomerization.arrow_forwardPropose a synthesis for (Z)-9-tricosene (muscalure), the sex pheromone for the common housefly (Musca domestica), starting with acetylene and haloalkanes as sources of carbon atoms.arrow_forwardSynthesize the following compound from benzonitrile (C6H5CN):arrow_forward
- Para-substituted product was produced when phenol reacts with cyclohexanecarbonyl bromide in the presence of AIB13. -Br Cyclohexanecarbonyl bromide (i) Outline the mechanism for this reaction. (ii) Draw the alternative substituted product formed.arrow_forward1. How would you prepare the following alkyl halides from the appropriate alcohols? (a) ÇI (b) Br CH3 CH3CH3 CH;CHCH,CHCH3 ČH3 2. Predict the products of the following reaction: (a) он CH3 (b) H2C CH;CH,CHCH,CHCH, Per, socl2. PBr3 ? H3Carrow_forwardThe reaction of 1-bromopropane and sodium hydroxide in ethanol occurs by an SN2mechanism. What happens to the rate of this reaction under the following conditions?(a) The concentration of NaOH is doubled.(b) The concentrations of both NaOH and 1-bromopropane are doubled.(c) The volume of the solution in which the reaction is carried out is doubled.arrow_forward
- Give reasons for the following: (i) p-nitrophenol is more acidic than p-methylphenol. (ii) Bond length of C—O bond in phenol is shorter than that in methanol. (iii) (CH3)3C—Br on reaction with sodium methoxide (Na+ _OCH3) gives alkene as the main product and not an ether.arrow_forward(c)Show step by step how to synthesize methoxybenzene from benzene.arrow_forwardFor each alkane, which mono brominated derivatives could you form in good yield by free-radical bromination?(a) cyclopentane (b) methylcyclopentane(c) 2-methylpentane (d) 2,2,3,3-tetramethylbutanearrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

