
Concept explainers
(a)
Interpretation:
The steps required to obtain the pure samples of the given compound from the starting material are to be stated.
Concept introduction:
The rearrangement in which acyl azides
Answer to Problem 23.60AP
The reaction route for the synthesis of the given product is shown below.
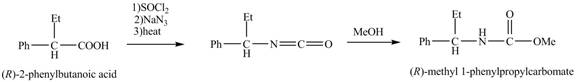
Explanation of Solution
The separation of enantiomers occurs in three steps.
In the first step, the two enantiomers,
The separation of the two enantiomers is shown below.
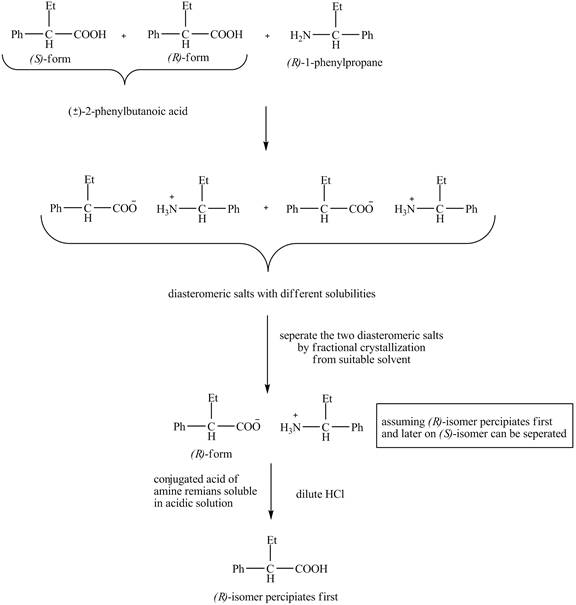
Figure 1
The formation of the given product follows the curtius rearrangement. The reaction follows a concerted mechanism, a reaction in which the bond formation and bond dissociation occurs in a single step.
The
The reaction for the formation of
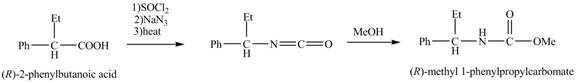
Figure 2
The desired product,
(b)
Interpretation:
The steps required to obtain the pure samples of the given compound from the starting material are to be stated.
Concept introduction:
An ester is formed by the reaction of carboxylic with alcohol in the presence of an acid catalyst. This reaction is known as Esterification.
Ester is the
Answer to Problem 23.60AP
The reaction route for the synthesis of the given product is shown below.
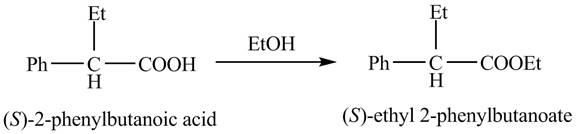
Explanation of Solution
The
The reaction of the formation of
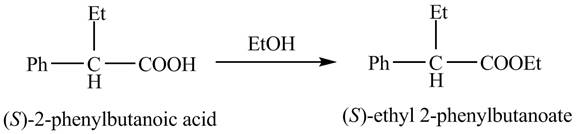
Figure 3
The formation of
(c)
Interpretation:
The steps required to obtain the pure samples of the given compound from the starting material are to be stated.
Concept introduction:
Hofmann rearrangement reaction is the conversion reaction of the primary amide to a primary amine with the loss of one carbon chain. The reaction requires basic conditions. The reaction of sodium hydroxide with bromine forms sodium hypobromite as an intermediate in situ.
Answer to Problem 23.60AP
The reaction route for the synthesis of the given product is shown below.
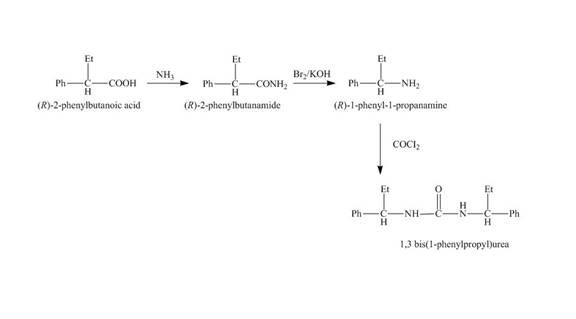
Explanation of Solution
The isomer,
The reaction of the formation of
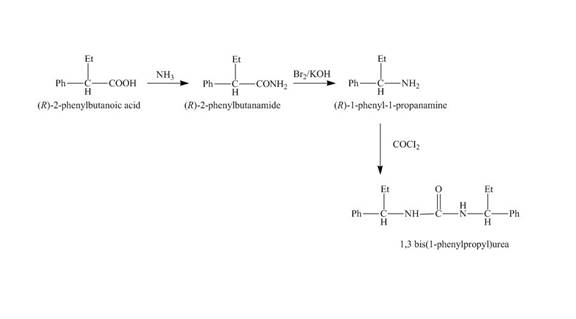
Figure 4
The formation of
(d)
Interpretation:
The steps required to obtain the pure samples of the given compound from the starting material are to be stated.
Concept introduction:
Hofmann rearrangement reaction is the conversion reaction of the primary amide to a primary amine with the loss of one carbon chain. The reaction requires basic conditions. The reaction of sodium hydroxide with bromine forms sodium hypobromite as an intermediate in situ.
Answer to Problem 23.60AP
The reaction route for the synthesis of the given product is shown below.
Explanation of Solution
The isomer,
The reaction of the formation of

Figure 5
The formation of
(e)
Interpretation:
The steps required to obtain the pure samples of the given compound from the starting material are to be stated.
Concept introduction:
Hofmann rearrangement reaction is the conversion reaction of the primary amide to a primary amine with the loss of one carbon chain. The reaction requires basic conditions. The reaction of sodium hydroxide with bromine forms sodium hypobromite as an intermediate in situ.
Answer to Problem 23.60AP
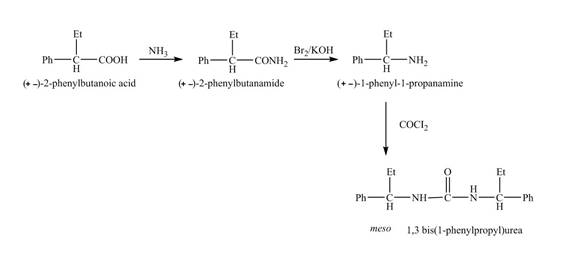
The reaction route for the synthesis of the given product is shown below.
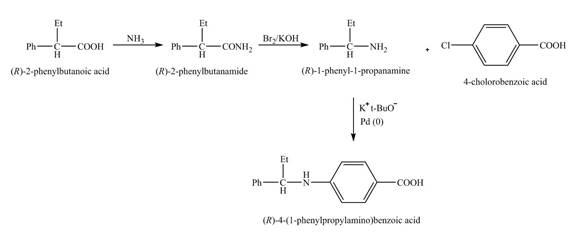
Explanation of Solution
The isomer,
The reaction of the formation of
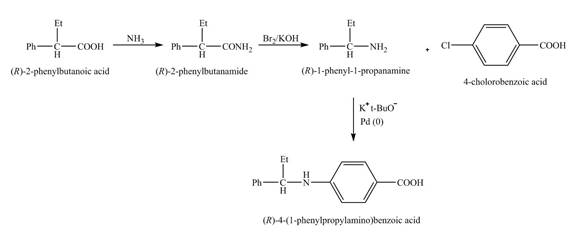
Figure 6
The formation of
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry
- Name each alkene and specify its configuration by the E,Z system. (Be sure to indicate double bond stereochemistry using (E)/(Z) notation. It is not necessary to use italics in writing compound names.) (a) (b) Brarrow_forwardProvide the reagents and solvents (where appropriate) needed to bring about the following transformations. (a) CI (b)arrow_forwardUsing cyclooctyne as your starting material, show how you would synthesize the following compounds. (Once you haveshown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)(a) cis-cyclooctenearrow_forward
- Name each alkene and specify its configuration by the E,Z system. (Be sure to indicate double bond stereochemistry using (E)/(Z) notation. It is not necessary to use italics in writing compound names.) (a) (b) (C) کو C1- Br لولهarrow_forward(a) CH3CHDOH (b) Show how to make these deuterium-labeled compounds, using CD3MgBr and D2O as your sources of deuterium, and any non-deuterated starting materials you wish. (a) CH3CH(OD)CD3 (d) Ph(CD3)2COD (b) CH3C(OH)(CD3)2 (c) CD3CH2CH2OHarrow_forwardChoose the more stable alkene in each of the following pairs. Explain your reasoning. (a) 1-Methylcyclohexene or 3-methylcyclohexene (b) Isopropenylcyclopentane or allylcyclopentane(d) (Z)-Cyclononene or (E)-cyclononene (e) (Z)-Cyclooctadecene or (E)-cyclooctadecenearrow_forward
- Using cyclooctyne as your starting material, show how you would synthesize the following compounds. (Once you haveshown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)(a) cis-cyclooctene (b) cyclooctane (c) trans-1,2-dibromocyclooctanearrow_forwardShow how you can synthesize the following compounds starting with benzene, toluene, and alcohols containing no morethan four carbon atoms as your organic starting materials. Assume that para is the major product (and separable fromortho) in ortho, para mixtures.(a) pentan-1-amine (b) N-methylbutan-1-amine(c) N-ethyl-N-propylbutan-2-amine (d) N-benzylpropan-1-amine(e)N N OH(e) (f) 3-propylaniline(g) 4-isobutylanilinearrow_forward(c) Consider the antiallergy medication loratadine (Claritin) shown below: Loratadine (Claritin) The reaction to form the portion of the molecule in the red circle is used in the synthesis of loratadine and many other pharmaceutical agents. (i) Propose a suitable starting material and reagents to form the portion of the molecule in the red circle. (ii) Clearly outline the mechanism by which the reaction takes place. In your answer, show how the intermediate ion is stabilized.arrow_forward
- Using cyclooctyne as your starting material, show how you would synthesize the following compounds. (Once you haveshown how to synthesize a compound, you may use it as the starting material in any later parts of this problem.)(a) cis-cyclooctene (b) cyclooctane (c) trans-1,2-dibromocyclooctane(d) cyclooctanone (e) 1,1-dibromocyclooctane (f) 3-bromocyclooctene(g) cyclooctane-1,2-dionearrow_forwardStarting from bromobenzene and any other reagents and solvents you need, show how you would synthesize the followingcompounds. Any of these products may be used as starting materials in subsequent parts of this problem.(a) 1-phenylpropan-1-olarrow_forward(c) Answer each of the questions below that relate to acetophenone: Xo (i) (ii) (iii) Draw the structure of the enol form of acetophenone. Give a stepwise mechanism for the conversion of acetophenone into its enol form. Show how each of the three compounds A, B and C below can be prepared from acetophenone. Explain clearly what reactants/reagents would be required in each case. odocor A B Br Carrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





