
Interpretation:
A mechanism for the dehydration of 4-methylcyclohexanol catalyzed by phosphoric acid needs to be explained.
Concept Introduction:
A dehydration reaction takes place after the removal of water molecule/s from the reactant molecule. In the mechanism of any organic reaction, negative charge always attacks on the positive center, and removal of good leaving group takes place. The stability of a leaving group depends on the stability of the molecule which can be shown by inductive, hyperconjugation or resonance effect.
Explanation of Solution
Below is the mechanism for the dehydration of 4-methyl cyclohexanol catalyzed by phosphoric acid:
In the first step, lone pair of electrons attack on the H atom which results in the formation positive charge on the O of the -OH group of 4-methyl cyclohexanol.
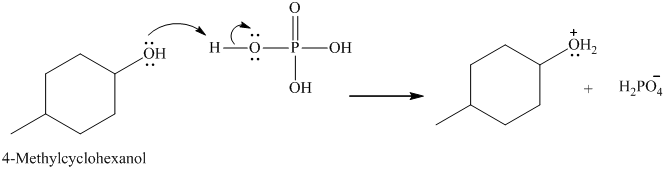
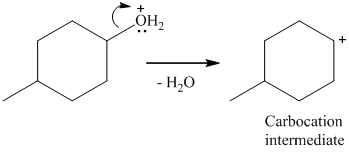
Here, the good leaving group is H2O. The derivative of hydronium ion removes a water molecule to form a carbocation intermediate.
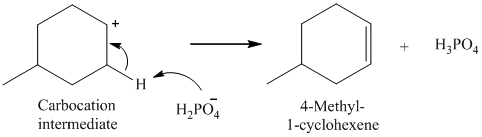
The nucleophile (H2PO4-) abstracts a proton from adjacent carbon of carbocation to form 4-methyl-1-cyclohexene.
Want to see more full solutions like this?
Chapter 22 Solutions
EBK A SMALL SCALE APPROACH TO ORGANIC L
- Nonconjugated , -unsaturated ketones, such as 3-cyclohexenone, are in an acid-catalyzed equilibrium with their conjugated , -unsaturated isomers. Propose a mechanism for this isomerization.arrow_forwardFollowing is the structural formula of the tranquilizer meparfynol (Oblivon). Propose a synthesis for this compound starting with acetylene and a ketone. (Notice the -yn- and -ol in the chemical name of this compound, indicating that it contains alkyne and hydroxyl functional groups.)arrow_forwardDescribe how 3-methyl-1-phenyl-3-pentanol can be prepared from benzene. You can use any inorganic reagents and solvents, and any organic reagents provided they contain no more than two carbons.arrow_forward
- 11) Synthesize 2-methyl-3-hydroxycyclohexone from cyclohexone, methyl iodide, and inorganic precursors.arrow_forwardWhat are the three alkenes formed in the acid-catalyzed dehydration of 2-pentanol? Propose the mechanism of their formation.arrow_forwardIn an attempt to synthesize compound C through a two-step process, a chemist discovered after completing the first step that they had inadvertently produced two distinct compounds, A and B. Upon examining the infrared spectroscopy (IR) results, it was observed that both A and B exhibited peaks indicative of a ketone and an ester group. Please provide the molecular structures of A and B. OEt NaOEt ΕΙΟ A B In a chemical experiment, they noticed that both components, A and B, from a combined sample turned into a new compound, C, during the following stage. The task is to determine what compound C looks like and explain how compound A or B changes into compound C through a reaction. Compound C should be the primary molecule containing carbon created in this process, not just a by-product. A B H3O+, H₂O, A Mechanism = сarrow_forward
- The ketone 2-heptanone has been identified as contributing to the odor of a number of dairy products, including condensed milk and cheddar cheese. Describe the synthesis of 2-heptanone from acetylene and any necessary organic and inorganic reagents.arrow_forwardAcid-catalyzed dehydration of 3-methyl-2-butanol gives three alkenes: 2-methyl-2- butene, 3-methyl-1-butene, and 2-methyl-1-butene. Propose a mechanism to account for the formation of each productarrow_forwarddescribe how steric and electronic effects influence the postion of equilibrium when the electrophilic center of an aldehyde or ketone is under nucleophilic attack.arrow_forward
- Methyl benzoate was exposed to a nitration test, although it was difficult to see the formation of a fragrant yellow solution. What produced this consequence?arrow_forwardWrite the mechanisms for the following reactions: Decanoic acid + Ethyl alcohol Propanoic acid + Ethyl alcohol Salicylic acid + Benzyl alcohol Decanoic acid + 3-methyl-1-butanolarrow_forwardHydration of aldehydes and ketones can be catalyzed by acid or base. Bases catalyze hydration by: protonating the carbonyl oxygen making the carbonyl group more electrophilic employing hydroxide ion, which is a better nucleophile than water making the carbonyl group less electrophilic shifting the equilibrium position of the reaction to favor productsarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


