
a)
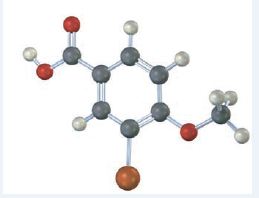
Interpretation:
The IUPAC name of the compound represented by the model shown is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain
To give:
The IUPAC name of the compound represented by the model shown.
Answer to Problem 17VC
The IUPAC name is 3-bromo-4-methoxybenzoic acid.
Explanation of Solution
The compound represented by the model is
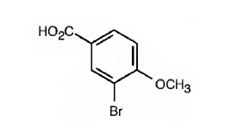
It is a benzoic acid derivative with a bromine atom on C3 and a methoxyl group on C4.
The IUPAC name of the compound represented by the model shown is 3-bromo-4-methoxybenzoic acid.
b)
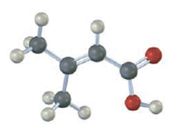
Interpretation:
The IUPAC name of the compound represented by the model shown is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The IUPAC name of the compound represented by the model shown.
Answer to Problem 17VC
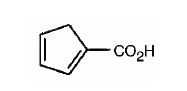
The IUPAC name of the compound represented by the model shown is 3-methyl-2-butenoic acid.
Explanation of Solution
The compound represented by the model is
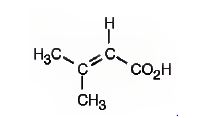
The compound is an unsaturated acid having a four carbon straight chain with a double bond between C2 and C3.
The IUPAC name of the compound represented by the model shown is 3-methyl-2-butenoic acid.
c)
Interpretation:
The IUPAC name of the compound represented by the model shown is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The IUPAC name of the compound represented by the model shown.
Answer to Problem 17VC
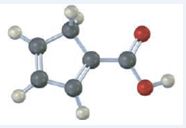
The IUPAC name of the compound represented by the model shown is cyclopenta-1,3-dienecarboxylic acid.
Explanation of Solution
The compound represented by the model is
The compound has a cyclopentadiene ring to which a carboxyl group is attached. The double bonds in the diene are in between C1 & C2 and C3 & C4.
The IUPAC name of the compound represented by the model shown is cyclopenta-1,3-dienecarboxylic acid.
d)
Interpretation:
The IUPAC name of the compound represented by the model shown is to be given.
Concept introduction:
The names of simple carboxylic acids which are derivatives of open-chain alkanes are arrived by replacing the terminal –e of the corresponding alkane name by –oic acid. The numbering starts from carboxyl carbon. Compounds with –COOH bonded to a ring are named using the suffix-carboxylic acid. The –COOH carbon in this case is not numbered as C1, instead the carbon to which it is attached is numbered as C1. As a substituent, the –COOH group is called as carboxyl group.
To give:
The IUPAC name of the compound represented by the model shown.
Answer to Problem 17VC
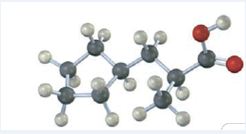
The IUPAC name of the compound represented by the model shown is (S)-3-cyclopentyl-2-methylpropanoic acid.
Explanation of Solution
The compound represented by the model is
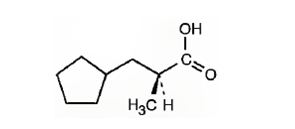
The compound is a carboxylic acid with a three carbon straight chin and has a methyl group attached to C2 and cyclopentyl group attached to C3. The compound is optically active as C2 is chiral. The groups of first highest, second highest and third highest groups when viewed away from the group of last priority are arranged in the anti-clockwise direction. Hence the compound has S stereochemistry.
The IUPAC name of the compound represented by the model shown is (S)-3-cyclopentyl-2-methylpropanoic acid.
Want to see more full solutions like this?
Chapter 20 Solutions
Organic Chemistry
- When the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forwardGive IUPAC names for the following substances (red = O, blue = N):arrow_forwardCH;CH-C-OH What is the correct IUPAC name for the compound above? O 1-ethylbenzoic acid O 2-methylbenzoic acid O 2-phenylpropanoic acid O 2-benzylpropanoic acidarrow_forward
- 19) What is the IUPAC name for this compound? CH3 H3C- -сн—сH,—CH2--с— он O 2-methyl-4-butanoic acid pentanoic acid 2-methyl butyric acid 3-methylbutanoic acid O 2-methylbutanoic acidarrow_forwardWhat is the IUPAC name for the compound shown? OH O 1-ethylcyclohexane-3-carboxylic acid O (2-ethyl)-1-cyclohexylmethanecarboxylic acid O 1-(2-ethyl)-cyclohexylmethanecarboxylic acid O 3-ethylcyclohexanoic acid O 3-ethylcyclohexanecarboxylic acidarrow_forwardGive an IUPAC name for the compound shown below:arrow_forward
- Which functional group among those shown below is that of a carboxylic acid? :0: :0: :0: tot tot C- -с-ӧн — С-N A B C D E O A OD O E B.arrow_forwardComplete the following reaction by supplying the missing portion indicated by a question mark: [0] CH;C–H ? Product v Ethanoic acid Propionic acid Propanone Ethanol Propanoic acid No reactionarrow_forwardName the following organic compound: CH₂ CH₂ H3C CH₂ OH O 2-oxo-5-pentanol O 4-oxo-1-pentanol O 5-hydroxy-2-pentanone O hydroxypentanone O 1-hydroxy-4-pentanonearrow_forward
- CH₂-CH₂-CH₂-C-OH butanoic acid CH₂ HO–C–CH,CHCH,—CHO 3-methylpentanoic acid Name each carboxylic acid. (a) HO CH₂ CH₂ 99 - 0 B) CHICHICIO CH, Draw a condensed structural diagram for each carboxylic acid (a) Hexanoic acid (b) 3-propyloctanoic acid Iarrow_forwardDraw the skeletal structure of the ester formed when carboxylic acid CH3CH2CH2CH2CO₂H is treated with ethanol (CH3CH₂OH) in the presence of H2SO4 Click and drag to start drawing a structure. Garrow_forward3) Which aa has the strongest acid side chain? a) b) H2N -CH-ö OH H,N -CH-C- OH CH2 ČH2 CH2 CH2 OH NH2 c) d) H2N -CH-ö OH H2N- CH OH CH2 CH-OH ČH3 OH 4) In order to oxidize an alcohol to aldehyde we use А) РСС В) НCL С) НЗО+arrow_forward

 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

