
Practice Problem 2.1
Propose structures for two constitutional isomers of cyclopentene that do not contain a ring.
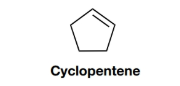
Interpretation:
The structuresfor two constitutional isomers of cyclopentene, which do not contain a ring, are to be proposed.
Concept introduction:
When two molecules have the same molecular formula, they are called isomers. If the connectivity of atoms is different, they are called constitutional isomers.
Constitutional isomers are the isomers in which the molecular formula remains the same but the arrangement of groups or atoms are different in structure.
In order to estimate the number of constitutional isomers of a particular compound, count the number of each atom and arranged all the atoms in different ways.
Answer to Problem 1PP
Solution:


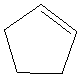
Given: The structure of cyclopentene is as follows:
Explanation of Solution
The structure of cyclopentane contains 5 carbon atoms and 8 hydrogen atoms. Thus, the molecular formula of cyclopenteneis
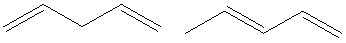
The molecular formula is
Hence, the two constitutional isomers of

Want to see more full solutions like this?
Chapter 2 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Chemistry
Chemistry: Structure and Properties (2nd Edition)
Organic Chemistry
Organic Chemistry (8th Edition)
Fundamentals of Heat and Mass Transfer
Chemistry & Chemical Reactivity
- PRACTICE PROBLEM 2.2 The compounds in each part below have the same (or similar) molecular weights. Which compound in cach part would you expect to have the higher boiling point? Explain your answers. (a) OH or (c) OH or HO (b) (CHN or A factor (in addition to polarity and hydrogen bonding) that affects the melting point of many organic compounds is the compactness and rigidity of their individual molecules. • Molecules that are symmetrical generally have abnormally high melting points. sert- Buryl alcohol, for example, has a much higher melting point than the other isomeric alcohols shown here: OH OH tert-Butyl alcohol (mp 25 "C) Butyl alcohol (mp -00 "C) Isobutyl alcohol sec-Butyl alcohol (mp 108 C) (mp-114 C) о оarrow_forwardWhich of the following is true about the carbon-carbon single bond in 1,3-butadiene when compared to the carbon-carbon single bond in ethane? (A) It is shorter than the carbon-carbon single bond of ethane. (B) The single bond in 1,3-butadiene has a higher %s character than in ethane The hydrogen atoms push the carbons closer using electrocyclic forces. Both A and B. (E) None of the above.arrow_forward(a) All Isomers have the same molecular formulae. Explain this further. Hint what does it mean to have the same molecular formulae? (b) What is Constitutional (structural) isomerism? Hint, isomers are different, so what is different between a pair of constitutional isomers (see the chart on the last page for examples). (c) What is Conformational isomerism?arrow_forward
- For each of the following compounds, identify all groups that would be considered substituents and then indicate the systematic name for each compound. (a) (b) (d) (e)arrow_forward2.34 Consider 1-bromopropane, CH3CH2CH2Br.(a) Draw a Newman projection for the conformation in which !CH3 and !Br are anti (dihedral angle 1808).(b) Draw Newman projections for the conformations in which !CH3 and !Br are gauche (dihedral angles 608 and 3008).(c) Which of these is the lowest energy conformation?(d) Which of these conformations, if any, are related by reflection?arrow_forward2.35 Consider 1-bromo-2-methylpropane and draw the following.(a) The staggered conformation(s) of lowest energy(b) The staggered conformation(s) of highest energyarrow_forward
- (B) Draw two chair conformations of the following substituted cyclohexane and identify the most stable conformation. Clearly show the axial and equatorial bonds.arrow_forward2.35 Write a structural formula for each of the following compounds: (a) 6-Isopropyl-2,3-dimethylnonane (b) 4-tert-Butyl-3-methylheptane (c) 4-Isobutyl-1,1-dimethylcyclohexane (d) sec-Butylcycloheptane (e) Cyclobutylcyclopentanearrow_forwardHO₂ H COOH COOH V POH HOT COOH cooff VI Ho HO COOH COOH VI H H COOH COOH VIIL OH OH Question: In the structures / examples abovke Identify V, VI, VII and VIII as Identical enantiomers, ciastereomes, stereo/cumers and/or structural isomers (there are 6 pairs 7 Comp eand to discover te I&T I TEN TEM TIMIN).arrow_forward
- The skeletal line formula for a branched alkene is shown below. (i) What is the molecular formula of this compound? (ii) How many carbon atoms are in the longest chain, ignoring the double bond? (iii) What is the longest chain incorporating both carbons of the double bond? (iv) How many substituents are on this chain? (v) Give the IUPAC name for this compound. [6]arrow_forward(d) Compound W has the molecular formula C.H.O. Compound W reacts when heated with ethanoic acid and a catalyst to produce a sweet-smelling liquid. (i) Give the name of the homologous series to which compound W belongs. (ii) Draw the structure of compound W. Show all of the atoms and all of the bonds. SVENTarrow_forward2.(a) Different conformations are found in 2-methylpentane. (i) Using Newman Projection, draw all the possible staggered and eclipsed conformations of pentane by referring to the bond rotation at C3-C4.(ii) Compare their stability and explain your answer.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





