
Concept explainers
Draw the predominant form of the following amino acids at physiological pH (7.4):
- a. Lysine
- b. Arginine
- c. tyrosine
(a)
Interpretation:
The predominant form of lysine at physiological
Concept introduction:
Acid-base properties of Amino acids:
Every amino acid has a carbonyl group and an amino group, and each group can exist in an acidic form or a basic form, depending on
The compound exists primarily in the acidic form in solutions that are more acidic than their
At
Answer to Problem 29P
The predominant form of lysine at physiological
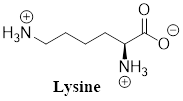
Explanation of Solution
The predominant form of glutamine at physiological

(b)
Interpretation:
The predominant form of arginine at physiological
Concept introduction:
Acid-base properties of Amino acids:
Every amino acid has a carbonyl group and an amino group, and each group can exist in an acidic form or a basic form, depending on
The compound exists primarily in the acidic form in solutions that are more acidic than their
At
Answer to Problem 29P
The predominant form of lysine at physiological
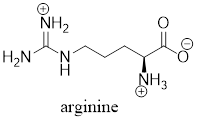
Explanation of Solution
The predominant form of lysine at physiological
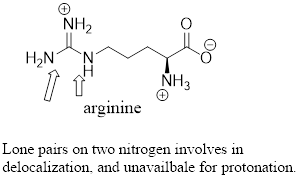
(c)
Interpretation:
The predominant form of tyrosine at physiological
Concept introduction:
Acid-base properties of Amino acids:
Every amino acid has a carbonyl group and an amino group, and each group can exist in an acidic form or a basic form, depending on
The compound exists primarily in the acidic form in solutions that are more acidic than their
At
Answer to Problem 29P
The predominant form of tyrosine at physiological
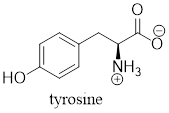
Explanation of Solution
The predominant form of tyrosine at physiological

Want to see more full solutions like this?
Chapter 17 Solutions
Essential Organic Chemistry (3rd Edition)
- Draw the predominant form for the aspartate amino acids at physiological pH (7.4):arrow_forward9) A COOH H₂N-C-H CH₂ CH₂ CH₂ CH₂ NH₂ 2.18 Coo H₂N-C-H CH₂ CH₂ CH₂ 8.95 Coo HN-C-H CH₂ CH₂ CH₂ 1 CH₂ NH₂ 16.53 What is this amino acid and the pl? a) This is arginine and the pl is 8.50 b) This is glutamine and the pl is 5.57 c) This is arginine and the pl is 9.62 Coo H₂N-C-H CH₂ 1 CH₂ CH₂ CH₂ NH₂arrow_forward16.6 Draw the structure for each of the following amino acids at physiological pH: a. lysine d. Tyr 16.7 Classify each of the amino acids in problem 16.5 as polar or nonpolar. If polar, indicate if the R group is neutral, acidic, or basic. Indicate if each is hydrophobic or hydrophilic. b. proline c. V 16.8 Classify each of the amino acids in problem 16.6 as polar or nonpolar. If polar, indicate if the R group is neutral, acidic, or basic. Indicate if each is hydrophobic or hydrophilic. Cibaarrow_forward
- 2. Calculate the pl for the amino acid shown below: (3/3) H₂N-CH- pKa = 9.60 CH₂ C- OH pKa = 3.65 -OH pKa = 1.88arrow_forward7. 8. 9. A Br Provide IUPAC name: 10. Arginine at pH=7 Draw structure OH leq H₂SO4 OH Provide missing reagent leq H₂SO4 KOH H₂O Enough base to raise pH ΝΗ IO Name the reaction: Provide product structure Name functional group Arginine at pH = 11 Draw the product(s) Draw structurearrow_forwardA. Draw the structure of the following amino acids and determine the Net Charge at pH - 6.5. 4. Phenylalanine 5. Asparaginearrow_forward
- 6.4 Draw the correct structure of the amino acid Glutamine (pl=5.7) at i Glutamine: R=CH-CH₂- -NH₂arrow_forwardC. Types of Steroid Hormones by Structure Steroid Hormone # of Carbon atom Structure 1. 2. 3arrow_forward25) Valine is one of the essential amino acids found in our bodies and used in the production of proteins. Valine has the condensed formula (CH3)2CH(NH2)CHCO2H. Draw the skeletal structure of valine as it would be represented at physiological pH (7.4). Answer:arrow_forward
- How many peptide bonds are present in the peptide below? HOT H. H. HO H.N N. H. N. H. Select one: a. 3 b.4arrow_forwardQ.1 Three given polypeptides (P, Q and R), of same size and same pl (7.2), have a single aspartic acid in each of them. In polypeptide P, the aspartic acid residue is on the surface. In polypeptide Q, it is surrounded by negatively charged residues, whereas in polypeptide R it is deeply buried in a hydrophobic core. pKa of the side chain carboxylate of free (in solution) aspartic acid is 3.65. The polypeptides are in a buller of pH 7.4. The clange in pKa of the aspartic acid side chain carboxylate in the polypeptides P, Q and R is likely to, Ans remain the same in P, modest increase in Q and greater increase in R X2. remain the same in all the three polypeptides X3. increase in P, decrease in Q and remain the same in R X 4. remain the same in P, increase Q and decrease in R.arrow_forward19.36 What type of interaction would you expect between the follow- ing groups in a tertiary structure? a. phenylalanine and isoleucine c. asparagine and tyrosine b. aspartate and histidine d. alanine and prolinearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





