
a)
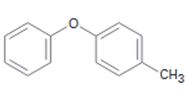
Interpretation:
The position and the ring in which the compound shown is expected to undergo electrophilic substitution is to be stated.
Concept introduction:
In
To state:
The position and the ring in which the compound shown is expected to undergo electrophilic substitution.
b)
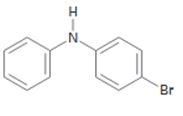
Interpretation:
The position and the ring in which the compound shown is expected to undergo electrophilic substitution is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions both -NH- and halogens are ortho and para directing as they stabilize the carbocation intermediates for these attacks. If two alternate options exist, the electrophile will enter into the more activated ring. The orientation of the incoming electrophile will be decided by the stronger of the two substituent groups already present.
To state:
The position and the ring in which the compound shown is expected to undergo electrophilic substitution.
c)
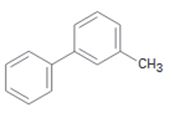
Interpretation:
The position and the ring in which the compound shown is expected to undergo electrophilic substitution is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions, both aryl and alkyl are ortho and para directing as they stabilize the carbocation intermediates for these attacks. If two alternate options exist, the electrophile will enter into the more activated ring. The orientation of the incoming electrophile will be decided by the stronger of the two substituent groups already present.
To state:
The position and the ring in which the compound shown is expected to undergo electrophilic substitution.
d)
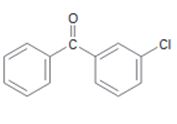
Interpretation:
The position and the ring in which the compound shown is expected to undergo electrophilic substitution is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions –C=O group is meta directing while halogens are ortho and para directing. If two alternate options exist the electrophile will enter into the more activated ring. The orientation of the incoming electrophile will be decided by the stronger of the two substituent groups already present.
To state:
The position and the ring in which the compound shown is expected to undergo electrophilic substitution.
Trending nowThis is a popular solution!

Chapter 16 Solutions
Organic Chemistry
- Provide the major product of each of the following reactions: What do all these reactions have in common? How do all reactions differ?arrow_forwardProvide the correct product for the following series of transformations. Hint: The product has a 1H NMR spectrum with only 2 resonances with relative H integrations of 3:1.arrow_forwardShow how to synthesize each of the following compounds using only a single nucleophilic aromatic substitution reaction. (a) NO, (b) (c) NO2 O,N ноarrow_forward
- On which ring and at what position/s would electrophilic aromatic substitution take place in the molecule below? Briefly account for your choice. A B CH3arrow_forwardIn each of the following reactions, the aromatic ring has just one chemically distinct, aromatic H, so a single electrophilic aromatic substitution will lead to just a single product. With this in mind, predict the product of each of these reactions. (a) (b) OzN. NO2 conc HNΟ, ? H,SO4, A conc HNO3 O,N `NO2arrow_forwardArrange these compounds in order of reactivity towards electrophilic aromaticsubstitution, with the most reactive compound listed firstarrow_forward
- Which carbon/s of the following compound will give the most electrophilic aromatic substitution product? You can choose more than one answer.arrow_forwardPredict the major product of the following reactions. If it is possible, write all stereoisomers.arrow_forwardIdentify the leaving group for a potential elimination of the following compounds. Compare the leaving group activities for A and K. Which of the following compounds cannot be subjected to elimination? Explain.arrow_forward
- What are the relative rates of the following nucleophilic substitution reaction below.arrow_forwardWhich of the following reactions are favorable or unfavorable? Explain why.arrow_forwardprovide a mechanism that explains the formation of the major product of the reaction. Please use resonance structures to explain the regiochemistry of this reaction and provide a detailed mechanism of this reaction.arrow_forward
