
Concept explainers
The terminal speed of ascent for a spherical
The time required for this bubble to rise
Whether this length of time is consistent with one’s observation.
Answer to Problem 76P
Explanation of Solution
Given:
The drag force on a moving sphere at a very low Reynolds number is
where,
Density of carbonated beverage,
Viscosity of carbonated beverage,
Diameter of the carbon dioxide bubble
Radius of the carbon dioxide bubble,
Formula used:
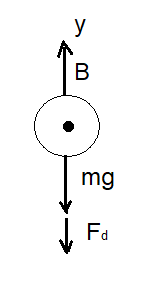
FIGURE: 1
The forces acting on the bubble before it reaches to its terminal speed are shown in the free body diagram (figure 1).
Applying Newton’s second law,
Where,
Under terminal speed conditions, acceleration
By using Archimedes principle, the buoyant force
Where,
Since
Where,
Mass of the gas bubble can be written as,
Substituting for
Where,
Volume of the carbon dioxide bubble which is spherical in shape can be written as,
Substituting for
Since
The rise time
Where,
Calculation:
Substituting the numerical values in equation
Substituting numerical values in equation
The time is less than a second. It seems reasonable.
Conclusion:
The terminal speed of ascent for a spherical
The time required for this bubble to rise
Want to see more full solutions like this?
Chapter 13 Solutions
Physics for Scientists and Engineers
- A car engine moves a piston with a circular cross-section of 73000.002cm in diameter a distance of 3.2500.001cm to compress the gas in the cylinder. (a) By what amount is the gas decreased in volume in cubic centimeters? (b) Find the uncertainty in this volume.arrow_forwardA straightforward method of finding the density of an object is to measure its mass and then measure its volume by submerging it in a graduated cylinder. What is the density of a 240-g rock 89.0 cm3 of water? (Note that the accuracy and practical applications of this technique are more limited than a variety of others that are based on Archimedes' principle.)arrow_forwardScurrilous con artists have been known to represent gold-plated tungsten ingots as pure gold and sell them at prices much below gold value but high above the cost of tungsten. With what accuracy must you be able to measure the mass of such an ingot in and out of water to tell that it is almost pure tungsten rather than pure gold?arrow_forward
- A water drop is observed to fall through a gas of density 0.001g/cm3 with a constant velocity of 980cm/s. What is the radius of the drop. The coefficient of viscosity of gas is 2*10 raise to power -4 poise.arrow_forward"The veloctice of fiue molecules are am/s, 3 m/s, 4 m/s, velocity I rool mean 5 m/s & 6 m/s opsp. Find mear square velocity of mobiles.arrow_forwardTo first approximation, we can ignore the flow of blood through the human circulatory system and treat it as a static fluid. Supposed you can measure a person’s blood pressure in both their anterior tibial artery (Pt, in the lower leg) and in their aorta (Pa, near the heart). Calculate the difference between Pt and Pa (i.e. Pa - Pt), in Pascals, when the person is lying down, and the person is standing. Use the fact that the distance between the two locations is approximately 1.35 m and the density of blood is 1060 kg/m3.arrow_forward
- The atmospheric pressure on earth is 101kPa. The surface area of the earth is related to its mean radius r which has a value of 6400km. Calculate the mass of the earth’s atmosphere, assuming g does not vary with height above the Earth’s surface. Calculate the number of nitrogen and oxygen molecules present on this atmosphere (take note: molecules).arrow_forwardCompute the average arterial pressure in the legs of an erect person, 130 cm below the heart.arrow_forwardAn important design consideration in two-phase pipe flow of solid-liquid mixtures is the terminal settling velocity below, which the flow becomes unstable and eventually the pipe becomes clogged. On the basis of extended transportation tests, the terminal settling velocity of a solid particle in the rest water given by VL = FL√2gD(S − 1), where FL is an experimental coefficient, g the gravitational acceleration, D the pipe diameter, and S the specific gravity of solid particle. What is the dimension of FL? Is this equation dimensionally homogeneous?arrow_forward
- ssm A patient recovering from surgery is being given fluid intravenously. The fluid has a density of 1030 kg/m², and 9.5 × 10-4 m³ of it flows into the patient every six hours. Find the mass 55. flow rate in kg/s.arrow_forwardThe average human has a density of 945 kg/m³ after inhaling and 1020 kg/m³ after exhaling. (a) Without making any swimming movements, what percentage of the human body would be above the surface in the Dead Sea (a body of water with a density of about 1230 kg/m³) in each of these cases? 24.06 after Your response is within 10% of the correct value. This may be due to roundoff error, or you could have a mistake in your calculation. Carry out all intermediate results to at least four-digit accuracy to minimize roundoff error.% inhaling 15.45 after Your response is within 10% of the correct value. This may be due to roundoff error, or you could have a mistake in your calculation. Carry out all intermediate results to at least four-digit accuracy to minimize roundoff error.% exhaling (b) Given that bone and muscle are denser than fat, what physical characteristics differentiate "sinkers" (those who tend to sink in water) from "floaters" (those who readily float)? (Select all that apply.)…arrow_forwardA light balloon is filled with 397 m3 of helium at atmospheric pressure. (a) At 0°C, the balloon can lift a payload of what mass? kg (b) In the table below, observe that the density of hydrogen is nearly half the density of helium. What load can the balloon lift if filled with hydrogen? | kg Densities of Some Common Substances at Standard Temperature (0°C) and Pressure (Atmospheric) Substance p(kg/m³) p(kg/m3) Substance 0.917 X 10 7.86 X 10 Air 1.29 Ice Aluminum 2.70 X 10 Iron Benzene 0.879 X 103 Lead 11.3 X 10 Copper 8.92 X 103 Mercury 13.6 X 103 Ethyl alcohol Fresh water 0.806 X 10% Oak 0.710 X 10 1.00 X 10 Oxygen gas 1.43 Glycerin 1.26 X 103 Pine 0.373 X 103 19.3 X 10 1.79 X 10-1 21.4 X 10 1.03 X 10 10.5 X 103 Gold Platinum Helium gas Seawater Hydrogen gas 8.99 X 10-2 Silverarrow_forward
 University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
