
Concept explainers
Interpretation:
The proton decoupled
Concept introduction:
The
Answer to Problem 13.63AP
The proton decoupled
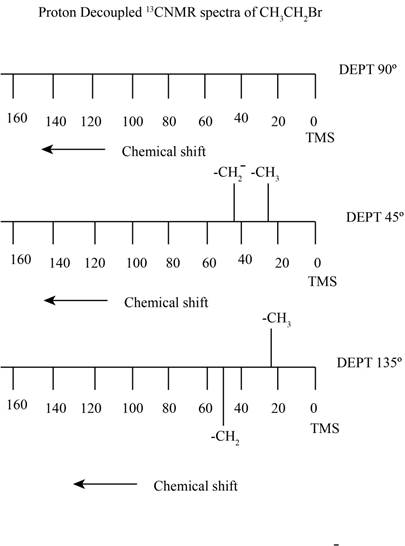
Explanation of Solution
The carbon atom exists in two isotopes
The relative intensity for the molecule in which no
The coefficient represents the number of atoms present of each element. Here the possibility of two
Here,
Here the possibility of two
The compound ethyl bromide contains two types of proton containing carbon. It gives rise to a quartet signal of
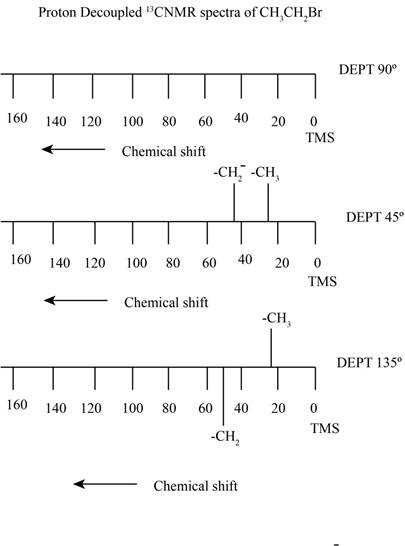
Figure 1
The proton decoupled
Want to see more full solutions like this?
Chapter 13 Solutions
Organic Chemistry
- N-H bonds in ammonia NH_3NH3 produce an IR absorbance at 3335 cm^-1. P-H bonds in phosphine PH_3PH3 produce an IR absorbance at 2327 ccm−1. Explain the difference in a single sentencearrow_forwardWhat IR frequencies would enable a chemist to distinguish between these molecules? CH;CH2OH and CH;CH2OCH;CH3 and CH, HCHCHCCIICH CH, d 3000-3100 cm-1 (=C-H)| 1620-1680 cm-1 (C=C) 3200-3400 cm-1 (OH) || 3000-3100 cm-1 (=C-H)arrow_forwardHow do the following factors affect absorption frequencies? O-H vs O-D. CH stretch, hybrid orbitals used by carbonarrow_forward
- A student takes an IR spectrum of an unknown compound. The IR spectrum shows significant stretches at 3159 cm³¹ (m, sh), 3089 cm ¹ (m, sh), 3000 cm ¹ (m, sh), 2997 cm-¹ (m, sh), 1620 cm-¹ (s, sh), 1494 cm-¹ (s, sh), and 1210 cm-¹ (s, sh). Which possible compound is it? Key: S = Strong, M = Medium, W = Weak, Sh = Sharp, Br = Broad Anisole Aniline O Acetanilide O Acetone O Phenolarrow_forwardThe mass spectrum of n-octane shows a prominent molecular ion peak (m>z 114). There is also a large peak at m>z 57,but it is not the base peak. The mass spectrum of 3,4-dimethylhexane shows a smaller molecular ion, and the peak atmass 57 is the base peak. Explain these trends in abundance of the molecular ions and the ions at mass 57, and predict theintensities of the peaks at masses 57 and 114 in the spectrum of 2,2,3,3-tetramethylbutane.arrow_forwardThe two compounds 1,2-dibromobenzene and 1 3-dibromobenzene both have the molecular formula C6H4Brz. a)Draw the chemical structures of the two molecules. b)Can these two molecules be distinguished by 1H NMR spectroscopy? Give reasons for your answer and describe the number of proton environments and their splitting for both molecules. Can these two molecules be distinguished by 13C NMR spectroscopy? Give reasons for your answer. Can these two molecules be distinguished by IR spectroscopy? Give reasons for your answer and describe the main IR signals (with frequencies) you expect to see for both molecules. Can these two molecules be distinguished by mass spectrometry? Give reasons for your answer.arrow_forward
- The mass spectra of two very stable cycloalkanes both show a molecular ion peak at m/z = 98 . One spectrum shows a base peak at m/z = 69, the other shows a base peak at m/z = 83. Identify the cycloalkane.arrow_forwardPredict the spin-spin splitting that you would expect to observe in the ¹H NMR spectra of the compound. H₂ CH3 H3C 1 Select the splitting pattern of the signal observed from the hydrogens on carbon 1. quintet quartet O singlet doublet O triplet FUN Select the splitting pattern of the signal observed from the hydrogens on carbon 2. O singlet doublet quartet triplet quintetarrow_forwardG Some absorptions may be weak but can be identified. Consider 4-tert-butylbenzonitrile and locate the four absorptions on the spectrum as each corresponds to the molecular bond. %T 100 80 60 40 20 2000 3000 CEN 2000 CC 1500 Wavenumberlcm-1] 1000 500 400 Identify these absorptions: C=N sp² C-H sp³ C-H C=C 2 EN ✓ 1arrow_forward
- The mass spectrum of an alkene, C8H16, exhibits a peak at m/z = 41. Draw two isomers that are consistent with these data.arrow_forwardThe following NMR and IR spectra were obtained for a molecule with molecular formula C2H4O2. Draw the structure of the molecule that is consistent with the two spectra. Note. In the NMR spectrum, s stands for singlet. LOD C2H,O2 3H, s 3000 cm-1 1H, s 1720 cm-1 D 4000 3000 2000 1500 1000 HAVENUMBERI l 12 10 8 4 -2arrow_forwardPredict the masses and the structures of the most abundant fragments observed in the mass spectra of the followingcompounds. cyclohexyl isopropyl ether [cyclohexyl¬O¬CH(CH3)2]arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
