
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12, Problem 12.24SP
covered a synthesis of
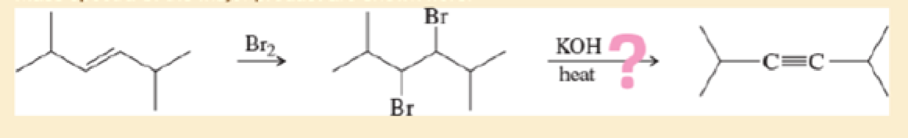
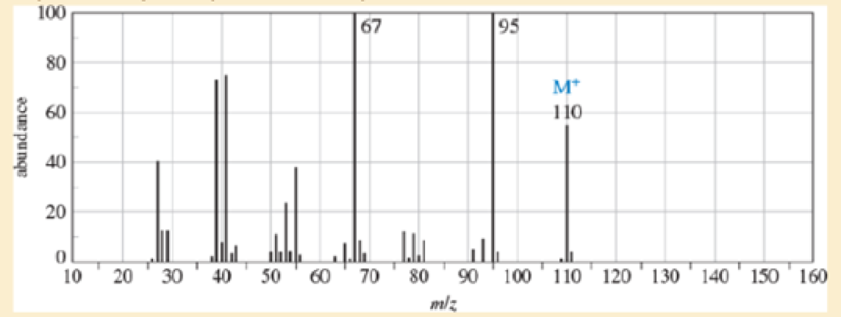
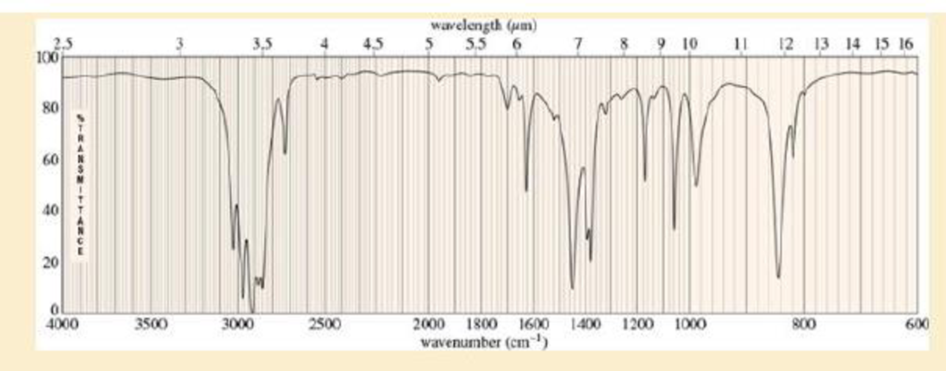
- a. Do the spectra confirm the right product? If not, what is it?
- b. Explain the important peaks in the IR spectrum.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
4. Circle the electrophilic and nucleophilic atoms in each substitution reaction below.
Provide the neutral organic product; where a stereocenter is created, use a wavy bond
to indicate a mixture of configurations or wedges/dashes for stereospecific reactions.
Also, provide the dominant reaction mechanism (Sn2 or SN1) somewhere in each box.
A.
Br
NaSEt
DMF
В.
ELOH
C.
.SO2NHNA
Cl
+
D.
NaH
Br-
HO,
DMF
8.
h.
OH
HCI (excess)
TSO
H₂SO4
A
Part IV. Predict the product X. Draw the complete, detailed mechanism (curved arrows) to show how the
tosylate will be eliminated to form molecule X and how molecule X will undergo an addition reaction to
form the final halohydrin-like products, using mechanistic skills gained in this course.
H₂O
150 °C
X
Br₂
LOH
Br
ibni
+
Br
Q₂H HOM S
One of the steps in the mechanism of the above reaction as shown below. Draw the structure of all the products of this
reaction.
com/mm/takeAssignCourt Rotivity.do?locator assignment-take
1
pt
ment
Eto
O
Eto
O
3. NaOH, H₂O, heat
4. HCI, H₂O, heat
One of the steps in the mechanism of the above reaction is shown below. Draw the structure of all of the products of this reaction.
O
OEt
O
1. Eto Na
2 Å
OH
You do not have to consider stereochemistry.
• Draw enolate anions in their carbanion form.
. You should include all products.
Ôno P
Ć
[References)
. Include counter-ions, e.g., Na, I, in your submission, but draw them in their own separate sketcher.
. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner.
. Separate multiple products using the + sign from the drop-down menu.
///-000- IF
Previous
Email Instructor
Chapter 12 Solutions
Organic Chemistry (9th Edition)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Additional Science Textbook Solutions
Find more solutions based on key concepts
The active ingredient in Tylenol and a host of other over-the-counter pain relievers is acetaminophen (C8H9NO2)...
Chemistry: Atoms First
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
Practice Exercise 1
Which of the following factors determines the size of an atom? a. the volume of the nucleus...
Chemistry: The Central Science (13th Edition)
Practice Problem ATTEMPT
Write the rate expressions for each of the following reactions:
(a)
(b)
(c)
Chemistry
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach
4. 38 Strontium has four naturally occurring isotopes, with mass numbers 84, 86, 87, arid 88.
a. Write the atom...
Basic Chemistry (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 3. Provide the mechanism for the following rxn: ethanol heat Br a. CH3 CH3 NAOCH3, CH3OH Br b. D H OH H,SO4 с.arrow_forward3. Provide the product of the haloform reaction shown below, as well as a mechanism for its formation. 1. Br2, NaOH 2. H3O+arrow_forwardDraw the major E2 reaction product formed when cis-1-chloro-2-ethylcyclohexane (shown) reacts with hydroxide ion in DMSO. Select Draw Rings More Erase H. CH,CH3 H. H. HO H- H. CI DMSO H. H. H. H. H.arrow_forward
- 1. MgBr 1. Dry ether OH 2. Hао 2. Br AIB 3 AIBra d = Electrophilic addition g = SN1 Nucleophilic a = Proton transfer substitution h = SN2 Nucleophilic b = Lewis acid/base e = El Elimination substitution c = Radical chain f= E2 Elimination substitution Identify the mechanism by which each of the reactions above proceeds from among the mechanisms listed. Use the letters a - i for your answers. 1. 2. Submit Answer Retry Entire Group 8 more group attempts remainingarrow_forwardQ10. For each of the following reactions (a-b), predict whether the substitution is more likely to occur following an Sn1 or Sn2 mechanism; explain your reasoning. Draw the products of each nucleophilic substitution paying particular attention to the stereochemistry of carbon 1. a) Br, CH3 MeOH H3C b) Br NaOH 1 DMF* H3C *N,N-dimethylformamidearrow_forward1. What is the function of CH»Ch in the bromination reactions? Why can it fulfil this role? 2. In not more than three (3) sentences, explain why terminal alkynes are acidic. 3. What impurities are removed when acetylene gas is made to pass through an acidified solution of CuSO:? 4. Explain the difference in the rate of free radical bromination reactions of toluene and cyclohexane. 5. Give the reagent er chemical cempounde, Previde only Ehe reasents nu Would differentiate tefelarrow_forward
- 17. a. Label the reactive features, highlight the most reactive one, then highlight what it needs. Also, state if the reaction will start to create a carbocation, carbon radical, or carbanion, or will cause loss of aromatic character. If a carbocation, carbon radical, or carbanion starts to develop, label where that will occur. CH3 with Cl, and FeCl, CH2 CH3 b. Use mechanism arrows to illustrate the reaction that occurs. c. If applicable, use stabilization resources to deal with the carbocation, carbon radical, or carbanion that starts to develop during the reaction, and draw the structure of any resonance-stabilized intermediate. d. Continue labeling and diagramming the reaction until you find the major stable product(s). e. Finally, state the stereochemistry of the major product(s) and use either Fisher projection or perspective formula representations to illustrate that stereochemistry.arrow_forwardThe following compounds are major products of an elimination reaction with an alkyl halide. Determine the structure of the alkyl halide. (P.S. write the overall reaction mechanism). Tip: Remember your Markovnikov’s rule. Follow the progress of the reaction and look at the stability of the intermediate products and transition states to determine the minor and major products. ANSWER ONLY THE FIRST 3 LETTERS.arrow_forwardPart 3. Predict the SN2/Radical Product. Predict the major organic product for each of the following reactions. Be sure to show stereochemistry when appropriate. MeO h 14 ** NaSCH3 NBS/hv/CCI4 NaN3arrow_forward
- For the reaction sequence below, provide the correct reagents, intermediate or final product. Br You? Na enant.arrow_forwardb).. .. Under each potential product of this E2 elimination, write "major product," "minor product," or "not formed." Me Br H Me Me OH Me Ph Ph H Me H Et Me Me Me Ph Ph Me Me Etarrow_forwardOH 4. Propose a mechanism for the alkylation step in Question ii) and iii) above Show all steps with structures.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License