
(a)
Interpretation:
Whether the reaction of ethylene with
Concept introduction:
The reaction of
Answer to Problem 11.53AP
The reaction of ethylene with
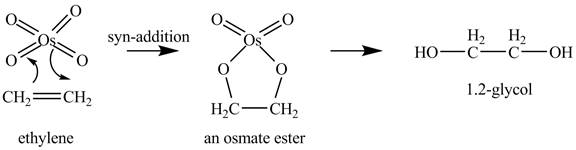
Explanation of Solution
Ethylene reacts with

Figure 1
Since, the product
The reaction of ethylene with
(b)
Interpretation:
Whether the reaction of
Concept introduction:
The reaction of alkenes with
Answer to Problem 11.53AP
The reaction of
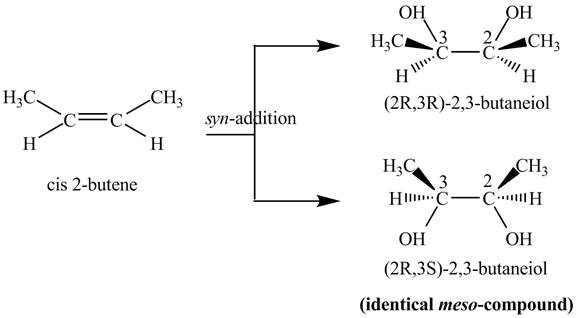
Explanation of Solution
The compound
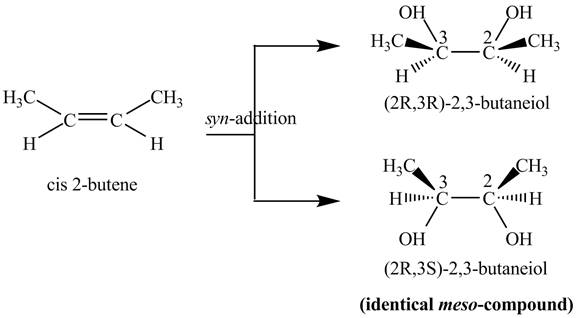
Figure 2
The two products of
The reaction of
(c)
Interpretation:
Whether the reaction of
Concept introduction:
The reaction of alkenes with
Answer to Problem 11.53AP
The reaction of
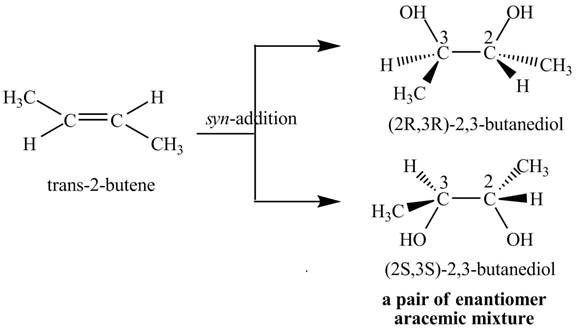
Explanation of Solution
The compound
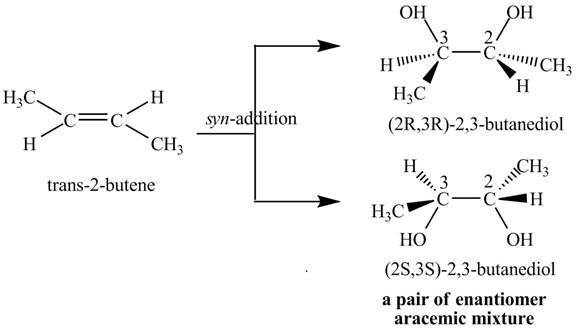
Figure 3
The products
The reaction of
(d)
Interpretation:
Whether the reaction of
Concept introduction:
The reaction of alkenes with
Answer to Problem 11.53AP
The reaction of
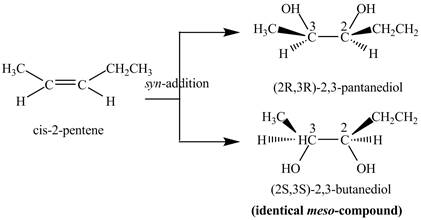
Explanation of Solution
The reaction of
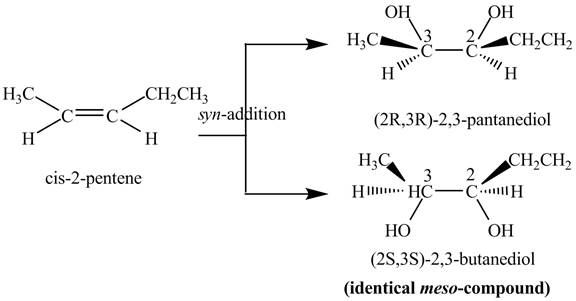
Figure 4
The two products:
The reaction of
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry
- Draw the structure of the organic product formed when 2,3-butanedione reacts with the following reagents. CH3 -C-C-CH3 | (a)H2/Ni (b)l2/NAOH 3Darrow_forwardShow how you would synthesize the following compounds, starting with acetylene and any compounds containing nomore than four carbon atoms.(a) hex-1-yne (b) hex-2-yne(c) cis-hex-2-ene (d) trans-hex-2-enearrow_forwardName the following aldehydes and ketones: (a) (b) CH2CH2CHO CH3CH2CCHCH3 CH3 Draw structures corresponding to the following names: (b) 4-Chloropentan-2-one (e) 3-Methylbut-3-enalarrow_forward
- Which compounds can be prepared in high yield by halogenation of an alkane? (a) 2-Chloropentane (b) Chlorocyclopentane (c) 2-Bromo-2-methylheptane (d) (R)-2-Bromo-3-methylbutane (e) 2-Bromo-2,4-trimethylpentane (f) Iodoethanearrow_forward6) Which is the organic product for the following reaction? (a) (b) (c) (d) LOH OH COOH OH OH COOH COOH KMnO4 H₂O (e) None of the above products will be formedarrow_forwardPredict the major products of the following reactions, (a) pent-1-ene + HCl b) 2-methylpropene + HCl(c) 1-methylcyclohexene + HI (d) 4-methylcyclohexene + HBrarrow_forward
- Br Each of the following deuterated bromoalkanes can be produced by treating an alkene with deuterium bromide (DBr). Draw the corresponding alkenes that could have been used. (a) Br D Br (b) Br 흐arrow_forwardAddition of HCI to the unsymmetrical alkene below could potentially give rise to two products. C2H5. CC=C: C2H5 CH3 (a) Draw the structures of the two potential carbocation intermediates. (b) State which carbocation is more stable. (c) Draw the structure of the major final product.arrow_forwardReaction of this bicycloalkene with bromine in carbon tetrachloride gives a trans dibro- mide. In both (a) and (b), the bromine atoms are trans to each other. However, only one of these products is formed. CH3 CH3 CH3 Br Br CH,Cl, + Br2 or Br Br (a) (b) Which trans dibromide is formed? How do you account for the fact that it is formed to the exclusion of the other trans dibromide?arrow_forward
- Name the following alkynes, and predict the products of their reaction with (1) H2 in the presence of a Lindlar catalyst and (2) H3O+ in the presence of HgSO4:arrow_forwardProvide the structure of the product(s) with stereochemisty if appropriate. If multiple products indicate major product or if products are formed in equal amounts. If there is no reaction expected explain why. (a) OH HIO4 (the protecting group is remained) CHO H HOH2C OH (b) CHO -ОН NaCN # HO -H H -OH CH₂OH (c) CHO HO- -H HO -H NH₂OH 張一 H -OH H -OH CH₂OH i) Ba(OH)2 ii) MeOH, H₂SO4 NaOAc Ac₂0 NaOMe MeOHarrow_forwardPredict the major products (including stereochemistry) when cis-3-methylcyclohexanol reacts with the following reagents. (a) concentrated HBr (b) TsCl/pyridine, then NaBrarrow_forward
