
Concept explainers
What two
a. an R-group and a hydroxyl group
b. an N–H group and a carbonyl group
c. an amino group and a hydroxyl group
d. an amino group and a carboxyl group
Introduction:
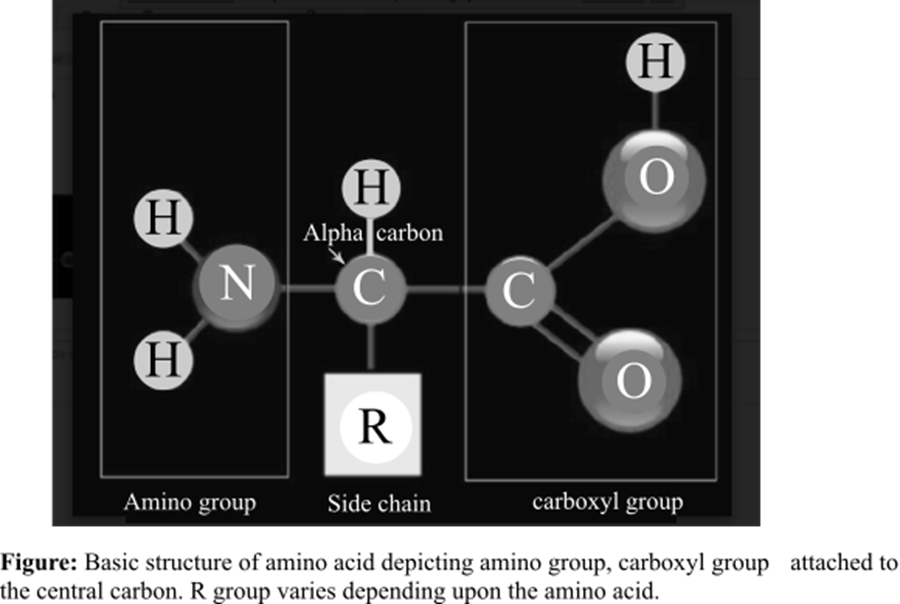
The amino acid is the basic structural unit of the proteins. There are total 20 amino acids found in the living system. At isoelectric point (pH or potential of hydrogen), an amino acid does not have any net charge. The basic structure of amino acids is shown below:
Answer to Problem 1TYK
Correct answer:
An amino group and a carboxyl group
Explanation of Solution
Explanation/Justification for the correct answer:
Option (d) is given as a carboxyl group along with an amino group. Functional groups of amino acids are responsible for bonding between two amino acids. The polypeptide chain consists of several amino acids. When a peptide bond is formed, a hydroxyl (–OH) group is lost from the carboxyl group of an amino acid and an H (hydrogen atom) from the amino group of another amino acid is also lost. This dehydration (loss of one H2O molecule) reaction results in a peptide bond. Hence, option (d) is correct.
Explanation for incorrect answers:
Option (a) is given as an R- group and a hydroxyl group. R group or side chain decides the identity of an amino acid, for example, glycine, which is the simplest amino acid, has a hydrogen atom as its R group. So, it is a wrong answer.
Option (b) is given as an N-H group and a carbonyl group. Any functional group, which has an O (oxygen) atom attached to the C (carbon) atom through double bond (-C=O) is called carbonyl group. COOH (carboxylic acid) is also a type of the carbonyl group. So, it is a wrong answer.
Option (c) is given as an amino group and a hydroxyl group. The carboxylic acid of the amino acid contains one hydroxyl group, which gets lost when the amino acid undergoes peptide bond formation with an amino group of another amino acid. So, it is a wrong answer.
Hence, options (a), (b), and (c) are incorrect.
The amino group and a carboxyl group are functional groups bounded to the central carbon of every free amino acid monomer.
Want to see more full solutions like this?
Chapter 3 Solutions
Biological Science (6th Edition)
- At the center of all 20 standard amino acids is what is termed the a-carbon that is covalently bonded with four other chemical groups. Which of these four chemical groups is not a normal component of all amino acids? A. an amino group B. a carboxyl group C. a side chain (R group) D. a methyl grouparrow_forwardWhich of these are considered “part” of each amino acid? A. Carboxyl acid group B. All of the options are correct C. R Group D. Amino grouparrow_forwardIdentify the components that are consistent between all 20 amino acids in the body. SELECT ALL THAT APPLY A. An amino group B. An Alpha-carbon C. A Carboxyl Group D. A Side chain R Grouparrow_forward
- Write the structure, identify the R groups and describe the chemical properties of these amino acids. A. Alanine B. Serine C. Sucrosearrow_forwardPredict the geometry around each carbon atom in the amino acid alanine.arrow_forwardWhich of the following correctly describes peptide bonds? * A. They are a special type of amide bond. B. They are very stable bonds. C. They form when water separates from an amino group and a carboxylic acid. D. They are a bond that shows resonance. E. All of these Which group consists only of amino acids with polar side chains? * A. serine, threonine, and leucine B. serine, threonine, and cysteine C. serine, threonine, and valine D. serine, threonine, and isoleucinearrow_forward
- Which of the following group(s) can be found in both an amino acid and a protein? a. Rgroup (side chain) only b. Free amino group and carboxyl group c. Free amino group, free carboxyl group and R groups d. Carbonyl group and free amino group and R groupsarrow_forwardGiven below is the structure of tallose.Answer the following questiona. what is the maximum number of stereoisomers can tallose have?b. how many chiral carbons does it have?c. what is the configuration of tallose is it D or L sugar?d. what type of monosaccharide is it?arrow_forwardChoose A if the statement is CORRECT B if the statement is WRONG This amino acid is/has: polar charged basic side chain 6. 7. 8. 9. aromatic side chain 10. K H H H N-C-C CH₂ CH ₂ CH₂ CH ₂ -Z N I NH₂ =O OHarrow_forward
- Draw the structural formula of a simple amino acid. What is the importance of the carboxyl group, amino group, and R group?arrow_forwardIdentify which of the following pairs of amino acid residues can have hydrogen bonding between their side chains. A. Alaine and Glycine B. Leucine and Isoleucine C. Valinc and Asparaginc D. Threonine and Tryrosinearrow_forwardThe structure of the dipeptide Gly-Asn is given by . The structures of the amino acids Gly and Asn are given below. NH2 H. ČH2 H3N-C-CO H. H3N-C-CO H. Asn Gly NH2 CH2 H H H2 C COO 2. H3N-C -C H. 1. H&N C C-NH C COO NH, NH2 CH2 H. H2 C C NH2 3. H3N-C C- -C COO 4. 0OC-C NH3" Click Save and Submit to save and submit. Click Save All Ansuwers to save all answers. MacBook Air esc 吕0 DII DD F1 F2 F3 F4 F5 F6 F7 F8 F9 @ 2$ & 1 2 4 5 7 8 Q W E R Y | A S D F G K C V N M < CO # 3 HICHarrow_forward
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education





