
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 13.8C, Problem 13.7P
Draw the NMR spectra you would expect for the following compound
- a. (CH3)2 CH—O—CH(CH3)2
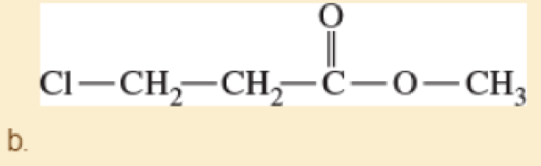
- c. Ph—CH(CH3)2
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The 'H NMR and 13C spectra of a compound with a molecular formula of C6H12O2 are shown below.
1. Name the compound in the textbox below.
2. Draw a possible structure for this compound.
1Η NMR
2H 2H
2H
3HPPM
3H
13C NMR
220
200
100
140
100
120
PPM
The structure of an ester could either be A or B:
CH3 O
O
CH3
T
||
-C-OCH3
CH3-C
CH3-C-O-C-CH3
CH3
CH3
A
B
Its ¹H NMR spectrum consists of two peaks at 80.9 and
83.6 (relative areas 3:1). Which compound is it? Describe
the spectrum that would be expected if it had been the
other ester.
Predict the structure of the molecule with molecular formula C8 H10 O using given information about its 1H NMR, 13C NMR, and IR.
Chapter 13 Solutions
Organic Chemistry (9th Edition)
Ch. 13.5A - In a 300-MHz spectrometer, the protons in...Ch. 13.5B - Prob. 13.2PCh. 13.6 - Determine the number of different kinds of protons...Ch. 13.6 - Prob. 13.4PCh. 13.7 - Draw the integral trace expected for the NMR...Ch. 13.7 - Prob. 13.6PCh. 13.8C - Draw the NMR spectra you would expect for the...Ch. 13.8D - Draw the NMR spectra you expect for the following...Ch. 13.8D - a. Assign protons to the peaks in the NMR spectrum...Ch. 13.8D - Prob. 13.10P
Ch. 13.8D - Two spectra are shown. Propose a structure that...Ch. 13.9 - Prob. 13.12PCh. 13.9 - The spectrum of trans-hex-2-enoic acid follows. a....Ch. 13.9 - Prob. 13.14PCh. 13.9 - Prob. 13.15PCh. 13.10 - Prob. 13.16PCh. 13.10 - If the imaginary replacement of either of two...Ch. 13.10 - Predict the theoretical number of different NMR...Ch. 13.11B - Prob. 13.19PCh. 13.11B - Prob. 13.20PCh. 13.11B - Prob. 13.21PCh. 13.11B - Prob. 13.22PCh. 13.11B - Prob. 13.23PCh. 13.11B - Prob. 13.24PCh. 13.12E - Draw the expected broadband-decoupled 13 C N M R...Ch. 13.12E - a. Show which carbon atoms correspond with which...Ch. 13.12E - Repeat Problem13-25, sketching the...Ch. 13.12F - Prob. 13.28PCh. 13.13 - A bottle of allyl bromide was found to contain a...Ch. 13.13 - A laboratory student was converting cyclohexanol...Ch. 13.14 - Sets of spectra are given for two compounds. For...Ch. 13 - An unknown compound has the molecular formula C 9...Ch. 13 - Prob. 13.34SPCh. 13 - Predict the approximate chemical shifts of the...Ch. 13 - Prob. 13.36SPCh. 13 - Prob. 13.37SPCh. 13 - Prob. 13.38SPCh. 13 - Prob. 13.39SPCh. 13 - Prob. 13.40SPCh. 13 - For each compound shown below. 1. sketch the 13 C...Ch. 13 - Prob. 13.42SPCh. 13 - Prob. 13.43SPCh. 13 - Prob. 13.44SPCh. 13 - Prob. 13.45SPCh. 13 - Prob. 13.46SPCh. 13 - A compound was isolated as a minor constituent in...Ch. 13 - Prob. 13.48SPCh. 13 - The three isomers of dimethylbenzene are commonly...Ch. 13 - a. Draw all six isomers of formula C 4 H 8...Ch. 13 - Prob. 13.51SPCh. 13 - Hexamethylbenzene undergoes free-radical...Ch. 13 - Each of these four structures has molecular...Ch. 13 - Prob. 13.54SPCh. 13 - Phenyl Grignard reagent adds to 2-methylpropanal...Ch. 13 - Prob. 13.56SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Propose a structural formula for each compound consistent with its 1H-NMR and 13C-NMR spectra. (a) C5H10O2 (b) C7H14O2 (c) C6 H12O2 (d) C7H12O4 (e) C4H7ClO2 (f) C4H6O2arrow_forwardHow would you use NMR (either 13C or 1H) to distinguish between the following pairs of isomers?arrow_forwardFollowing are 1H-NMR spectra for compounds G, H, and I, each with the molecular formula C5H12O. Each is a liquid at room temperature, is slightly soluble in water, and reacts with sodium metal with the evolution of a gas. (a) Propose structural formulas of compounds G, H, and I. (b) Explain why there are four lines between 0.86 and 0.90 for compound G. (c) Explain why the 2H multiplets at 1.5 and 3.5 for compound H are so complex.arrow_forward
- The compound whose 1H NMR spectrum is shown has the molecular formula C3H6Br2. Propose a structure.arrow_forwardPhenacetin, a substance formerly used in over-the-counter headache remedies, has the formula C10H13NO2. Phenacetin is neutral and does not dissolve in either acid or base. When warmed with aqueous NaOH, phenacetin yields an amine, C8H11NO, whose 1H NMR spectrum is shown. When heated with HI, the amine is cleaved to an aminophenol, C6H7NO. What is the structure of phenacetin, and what are the structures of the amine and the aminophenol?arrow_forwardAn unknown compound reacts with ethyl chloride and aluminum trichloride to form a compound that has the following 1H NMR spectrum. What is the structure of the compound?arrow_forward
- Using the following NMR data, and the molecular formula C9H120, select the best compound. NMR Data triplet 1.15 ppm (3H) quartet 2.75 ppm (2H) singlet 3.75 ppm (3H) split quartet 7.5 ppm (4H) Multiple choice options A: 1-ethyl-4-methoxybenzene B: 1-ethoxy-4-methylbenzene C: 1-bromo-4-methylbenzene D: 1-bromo-4-ethylbenzenearrow_forwardCompound A has molecular formula C7H7X. Its ¹H-NMR spectrum shows a singlet at 2.26 ppm and two doublets, one at 6.95 ppm and one at 7.28 ppm. The singlet has an integral of three and the doublets each have an integral of two. Its 13C- NMR shows five signals. The mass spectrum of A shows a peak at m/z 170 and another peak at m/z = 172; the relative height of the two peaks is 1:1 respectively. - Identify what atom X is, explaining your reasoning - Identify Compound A, explaining your reasoning Compound A is treated with a mixture of nitric and sulfuric acids to generate Compound B. The ¹H-NMR spectrum of B shows two singlets, one at 2.52 pm and one at 8.13 ppm. The 13C-NMR spectrum of B shows five signals. The mass spectrum of B shows a peak at m/z = 260 and another peak at m/z = 262; the relative height of the two peaks is 1:1 respectively. - Identify compound B, explaining your reasoning Compound B is treated with sodium ethoxide to generate compound C. The ¹H-NMR spectrum of C shows…arrow_forwardAn unknown compound has the molecular formula C7H14O, and its 1H NMR and 13C NMR spectra are shown below. Determine the structure of the unknown compound and draw it below. Note that there are no peaks above 3 ppm in the 1H NMR, and the numbers present on the 1H NMR are the integration values for each set of peaks.arrow_forward
- Using 1H NMR Spectroscopy to Distinguish Between Compounds How could 1H NMR spectroscopy be used to distinguish between compounds X and Y?arrow_forwardA ¹H NMR spectrum is shown for a molecule with the molecular formula of CsH10O2. Draw the structure that best fits this data. 10 pom aarrow_forwardCompound A has molecular formula C7H7X. Its 1H-NMR spectrum shows a singlet at 2.26 ppm and two doublets, one at 6.95 ppm and one at 7.28 ppm. The singlet has an integral of three and the doublets each have an integral of two. Its 13C-NMR shows five signals. The mass spectrum of A shows a peak at m/z = 170 and another peak at m/z = 172; the relative height of the two peaks is 1:1 respectively. - Identify what atom X is, explaining your reasoning - Identify Compound A, explaining your reasoning Compound A is treated with a mixture of nitric and sulfuric acids to generate Compound B. The 1H-NMR spectrum of B shows two singlets, one at 2.52 pm and one at 8.13 ppm. The 13C-NMR spectrum of B shows five signals. The mass spectrum of B shows a peak at m/z = 260 and another peak at m/z = 262; the relative height of the two peaks is 1:1 respectively. - Identify compound B, explaining your reasoning Compound B is treated with sodium ethoxide to generate compound C. The 1H-NMR spectrum of C shows…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY