From what we know today, what do the two Kekulé structures for benzene really represent? August Kekulé was the first person to propose a viable structure for benzene in 1865. Structures that can be separated at low enough temperatures (near absolute zero). Structures that are in a state of rapid equilibrium. O Structures that are in resonance. Structures that are conjugated trienes (--C=C--C%3DC--C3C--).
From what we know today, what do the two Kekulé structures for benzene really represent? August Kekulé was the first person to propose a viable structure for benzene in 1865. Structures that can be separated at low enough temperatures (near absolute zero). Structures that are in a state of rapid equilibrium. O Structures that are in resonance. Structures that are conjugated trienes (--C=C--C%3DC--C3C--).
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter6: Covalent Bonding
Section: Chapter Questions
Problem 77QRT
Related questions
Question

Transcribed Image Text:From what we know today, what do the two Kekulé structures for benzene really represent? August Kekulé was the first person to propose a viable
structure for benzene in 1865.
Structures that can be separated at low enough temperatures (near absolute zero).
Structures that are in a state of rapid equilibrium.
O Structures that are in resonance.
Structures that are conjugated trienes (--C=C--C%3DC--C3C--).
Expert Solution
Step 1
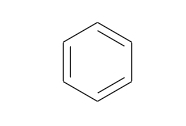
Friedrich August Kekule, in 1865, suggested that the benzene contain a ring of six carbon atom in which double bond between two carbon and single bond between two carbon atoms are alternatively arranged.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning