2. Using the reaction mixture in Problem 1, a student found that it took 510 seconds for the color of the 1₂ to disappear. a. What was the rate of the reaction? Hint: First find the initial concentration of I₂ in the reaction mix- ture, [1₂]o. Then use Equation 5. rate= rate = M/sec b. Given the rate from Part (a), and the initial concentrations of acetone, H* ion, and I₂ in the reaction mixture, write Equation 3 as it would apply to the mixture.
2. Using the reaction mixture in Problem 1, a student found that it took 510 seconds for the color of the 1₂ to disappear. a. What was the rate of the reaction? Hint: First find the initial concentration of I₂ in the reaction mix- ture, [1₂]o. Then use Equation 5. rate= rate = M/sec b. Given the rate from Part (a), and the initial concentrations of acetone, H* ion, and I₂ in the reaction mixture, write Equation 3 as it would apply to the mixture.
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 14.97QE: According to the Resource Conservation and Recovery Act (RCRA), waste material is classified as...
Related questions
Question
Please answer 2b highlighted. Explain
![1. In a reaction involving the iodination of acetone, the following volumes were used to make up the reac-
tion mixture:
a.
How many moles of acetone were in the reaction mixture? Recall that, for a component A, moles
A = MAX V, where MA is the molarity of A and V is the volume in liters of the solution of A that
was used.
5 mL 4.0 M acetone + 10 mL 1.0 M HCl + 10 mL 0.0050 M I₂ + 25 mL H₂O
M₁ =
b. What was the molarity of acetone in the reaction mixture? The volume of the mixture was 50 mL,
0.050 L, and the number of moles of acetone was found in Part (a). Again,
moles of A
V of soln. in liters
moles acetone
c. How could you double the molarity of the acetone in the reaction mixture, keeping the total volume
at 50 mL and keeping the same concentrations of H+ ion and I₂ as in the original mixture?
rate =
2. Using the reaction mixture in Problem 1, a student found that it took 510 seconds for the color of the 1₂ to
disappear.
M acetone
a. What was the rate of the reaction? Hint: First find the initial concentration of I₂ in the reaction mix-
ture, [1₂]o. Then use Equation 5.
rate =
C. What are the unknowns that remain in the equation in Part (b)?
b. Given the rate from Part (a), and the initial concentrations of acetone, H* ion, and I₂ in the reaction
mixture, write Equation 3 as it would apply to the mixture.
M/sec](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F21b2424c-a4ba-45d0-aa84-edf812370fbb%2F81e4bb8d-8979-4a56-a615-4714f22655f6%2Fucezuxh_processed.jpeg&w=3840&q=75)
Transcribed Image Text:1. In a reaction involving the iodination of acetone, the following volumes were used to make up the reac-
tion mixture:
a.
How many moles of acetone were in the reaction mixture? Recall that, for a component A, moles
A = MAX V, where MA is the molarity of A and V is the volume in liters of the solution of A that
was used.
5 mL 4.0 M acetone + 10 mL 1.0 M HCl + 10 mL 0.0050 M I₂ + 25 mL H₂O
M₁ =
b. What was the molarity of acetone in the reaction mixture? The volume of the mixture was 50 mL,
0.050 L, and the number of moles of acetone was found in Part (a). Again,
moles of A
V of soln. in liters
moles acetone
c. How could you double the molarity of the acetone in the reaction mixture, keeping the total volume
at 50 mL and keeping the same concentrations of H+ ion and I₂ as in the original mixture?
rate =
2. Using the reaction mixture in Problem 1, a student found that it took 510 seconds for the color of the 1₂ to
disappear.
M acetone
a. What was the rate of the reaction? Hint: First find the initial concentration of I₂ in the reaction mix-
ture, [1₂]o. Then use Equation 5.
rate =
C. What are the unknowns that remain in the equation in Part (b)?
b. Given the rate from Part (a), and the initial concentrations of acetone, H* ion, and I₂ in the reaction
mixture, write Equation 3 as it would apply to the mixture.
M/sec
Expert Solution
Step 1
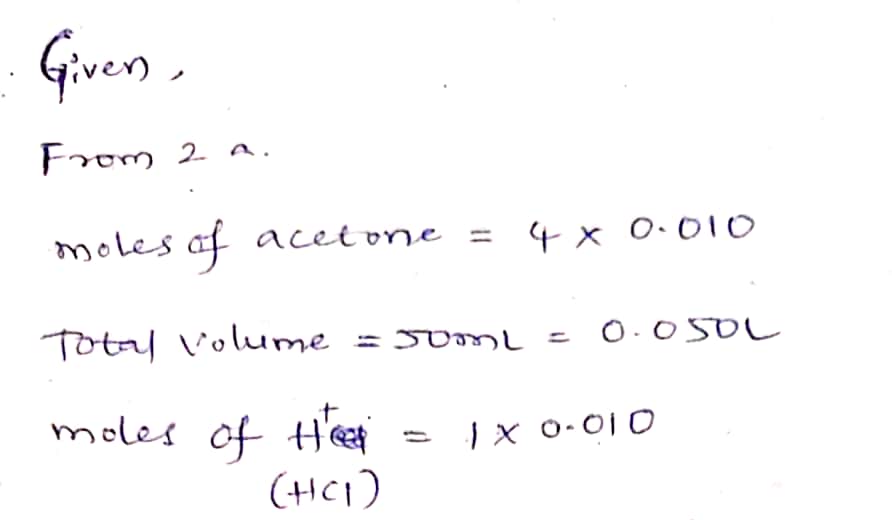
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning